Maker of highly addictive OxyContin says it will stop incentivizing doctors to push its deadly drugs
02/16/2018 / By Tracey Watson

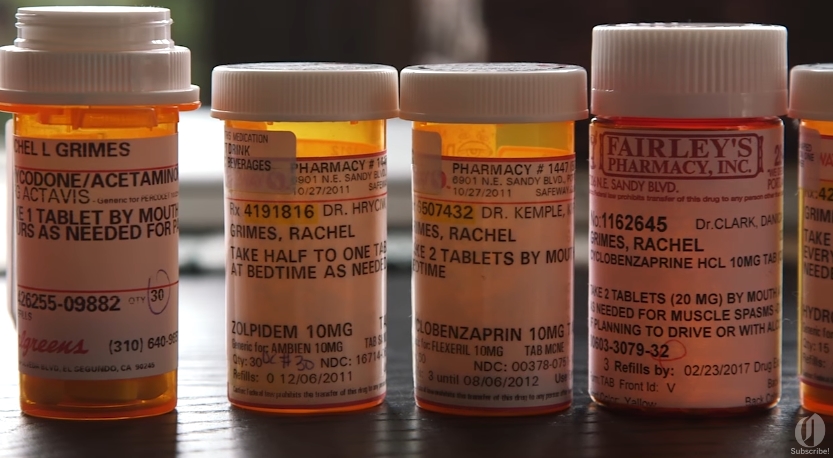
The opioid crisis has reached epidemic proportions in this country, with the number of deaths quadrupling since 2000, and around 115 people dying from overdoses each day. President Trump has declared the opioid crisis a national health emergency, and high schools across the country have started stocking up on the drug Narcan to try to save lives in case of an accidental overdose on school premises.
The Centers for Disease Control and Prevention (CDC) estimates that when you consider direct healthcare costs, lost productivity, criminal prosecution and addiction treatment, this crisis costs the United States around $78.5 billion annually.
Now, Big Pharma giant Purdue, the manufacturer of OxyContin, the world’s bestselling opioid drug, has vowed to stop marketing the drug to healthcare professionals.
Several pharmaceutical manufacturers, including Purdue, have admitted to having entire teams of sales people, as well as “front groups” and “key opinion leaders,” pushing the drug as a mainstream option for chronic, long-term pain, even though the drug was designed for cancer patients and others suffering severe pain who only need pain relief for a limited period of time.
As reported by Circa, when the drug was first released in 1995, it was hailed as a breakthrough in the treatment of severe pain, but users quickly learned that they could abuse the drug to get a high:
It worked over 12 hours to maintain a steady level of oxycodone in patients suffering from a wide range of pain ailments. But some users quickly discovered they could get a heroin-like high by crushing the pills and snorting or injecting the entire dose at once. In 2010 Purdue reformulated OxyContin to make it harder to crush and stopped selling the original form of the drug.
The manufacturer did not discontinue making the drug, of course, and went on to pitch it to healthcare professionals as a low-risk wonder drug for the treatment of pain. Purdue also later admitted to minimizing the addiction risk to doctors, who went on to prescribe it for many off-label uses, directly resulting in the crisis the country is now faced with. (Related: Learn more about what caused this crisis and what is being done to cope with it at Opioids.news.)
In the meantime, Purdue has been raking in billions in profits each year.
It is unlikely that the company has altruistic reasons for agreeing to stop lying to doctors about the risks and benefits of OxyContin. It is far more likely that the multiple lawsuits it has been inundated with has led to this change of heart.
While it is certainly better that Purdue is now no longer going to pitch propaganda to the healthcare community and has halved its sales staff to that end, experts believe that other pharmaceutical companies will have to follow suit before any real progress can be made in turning the tide of the opioid crisis.
“It is difficult to promote more cautious prescribing to the medical community because opioid manufacturers promote opioid use,” said Dr. Andrew Kolodny, director of opioid policy research at Brandeis University, and expert adviser in several of the lawsuits pending against Big Pharma.
While Purdue is a major producer of opioids, other big players like Johnson & Johnson and Allergan would have to make the same commitment to stopping the propaganda machine for any real progress to be made. (Related: Didn’t they take an oath not to do harm? Opioids are coming from doctors’ offices, not the ER.)
And while Purdue has promised to make changes in the U.S., it is making no such commitment regarding the international market.
“They are still doing this abroad,” Kolodny noted. “They are following the same playbook that they used in the United States.”
Circa noted that while Purdue Pharma only operates under that name in the United States, it is associated with two other pharmaceutical companies, Mundipharma and Napp, overseas. Purdue has distanced itself from these producers, saying that they operate independently and according to local regulations.
So, no real change of heart, then. They’ve simply turned their attention to the next lucrative market.
Sources include:
Tagged Under: addiction, Big Pharma, corruption, doctors, drug marketing, greed, opioid crisis, Opioids, oxycontin, propaganda, Purdue Pharma